
- Current
- Browse
- Collections
-
For contributors
- For Authors
- Instructions to authors
- Article processing charge
- e-submission
- For Reviewers
- Instructions for reviewers
- How to become a reviewer
- Best reviewers
- For Readers
- Readership
- Subscription
- Permission guidelines
- About
- Editorial policy
Search
- Page Path
- HOME > Search
- Basic Research
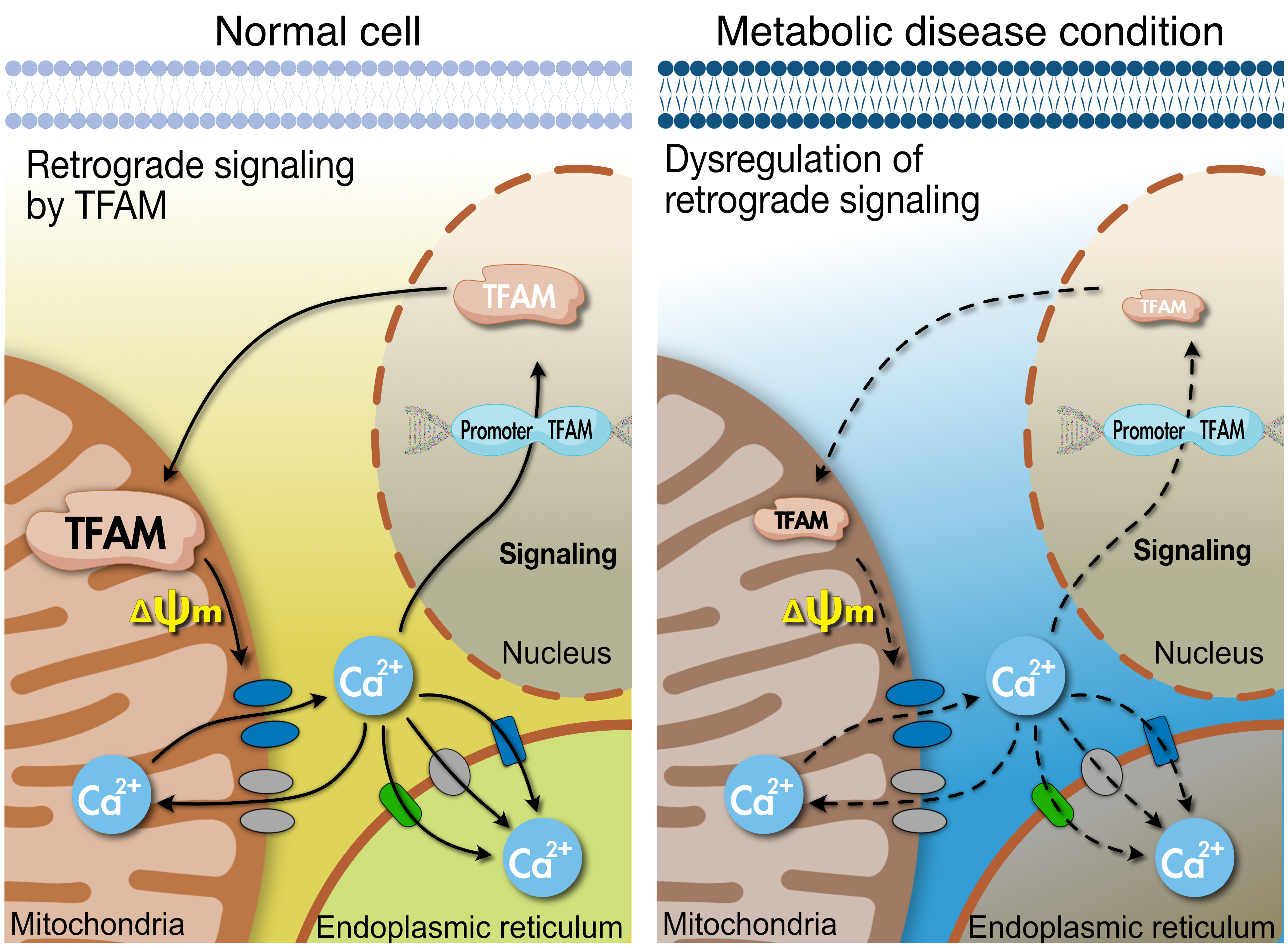
- Mitochondrial TFAM as a Signaling Regulator between Cellular Organelles: A Perspective on Metabolic Diseases
- Jin-Ho Koh, Yong-Woon Kim, Dae-Yun Seo, Tae-Seo Sohn
- Diabetes Metab J. 2021;45(6):853-865. Published online November 22, 2021
- DOI: https://doi.org/10.4093/dmj.2021.0138
- 6,700 View
- 275 Download
- 14 Web of Science
- 15 Crossref
-
 Graphical Abstract
Graphical Abstract
 Abstract
Abstract
 PDF
PDF PubReader
PubReader  ePub
ePub 
- Tissues actively involved in energy metabolism are more likely to face metabolic challenges from bioenergetic substrates and are susceptible to mitochondrial dysfunction, leading to metabolic diseases. The mitochondria receive signals regarding the metabolic states in cells and transmit them to the nucleus or endoplasmic reticulum (ER) using calcium (Ca2+) for appropriate responses. Overflux of Ca2+ in the mitochondria or dysregulation of the signaling to the nucleus and ER could increase the incidence of metabolic diseases including insulin resistance and type 2 diabetes mellitus. Mitochondrial transcription factor A (Tfam) may regulate Ca2+ flux via changing the mitochondrial membrane potential and signals to other organelles such as the nucleus and ER. Since Tfam is involved in metabolic function in the mitochondria, here, we discuss the contribution of Tfam in coordinating mitochondria-ER activities for Ca2+ flux and describe the mechanisms by which Tfam affects mitochondrial Ca2+ flux in response to metabolic challenges.
-
Citations
Citations to this article as recorded by- Targeted metabolomics reveals the aberrant energy status in diabetic peripheral neuropathy and the neuroprotective mechanism of traditional Chinese medicine JinMaiTong
Bingjia Zhao, Qian Zhang, Yiqian He, Weifang Cao, Wei Song, Xiaochun Liang
Journal of Pharmaceutical Analysis.2024; 14(2): 225. CrossRef - Mitochondrial damage‐associated molecular patterns: A new insight into metabolic inflammation in type 2 diabetes mellitus
Yan Wang, Jingwu Wang, Si‐Yu Tao, Zhengting Liang, Rong xie, Nan‐nan Liu, Ruxue Deng, Yuelin Zhang, Deqiang Deng, Guangjian Jiang
Diabetes/Metabolism Research and Reviews.2024;[Epub] CrossRef - Altered Energy Metabolism, Mitochondrial Dysfunction, and Redox Imbalance Influencing Reproductive Performance in Granulosa Cells and Oocyte During Aging
Hiroshi Kobayashi, Chiharu Yoshimoto, Sho Matsubara, Hiroshi Shigetomi, Shogo Imanaka
Reproductive Sciences.2024; 31(4): 906. CrossRef - When Our Best Friend Becomes Our Worst Enemy: The Mitochondrion in Trauma, Surgery, and Critical Illness
May-Kristin Torp, Kåre-Olav Stensløkken, Jarle Vaage
Journal of Intensive Care Medicine.2024;[Epub] CrossRef - Attenuating mitochondrial dysfunction and morphological disruption with PT320 delays dopamine degeneration in MitoPark mice
Vicki Wang, Kuan-Yin Tseng, Tung-Tai Kuo, Eagle Yi-Kung Huang, Kuo-Lun Lan, Zi-Rong Chen, Kuo-Hsing Ma, Nigel H. Greig, Jin Jung, Ho-II Choi, Lars Olson, Barry J. Hoffer, Yuan-Hao Chen
Journal of Biomedical Science.2024;[Epub] CrossRef - Effects of the anti-inflammatory drug celecoxib on cell death signaling in human colon cancer
Ryuto Maruyama, Yuki Kiyohara, Yasuhiro Kudo, Tomoyasu Sugiyama
Naunyn-Schmiedeberg's Archives of Pharmacology.2023; 396(6): 1171. CrossRef - gp130 Activates Mitochondrial Dynamics for Hepatocyte Survival in a Model of Steatohepatitis
Daria Shunkina, Anastasia Dakhnevich, Egor Shunkin, Olga Khaziakhmatova, Valeria Shupletsova, Maria Vulf, Alexandra Komar, Elena Kirienkova, Larisa Litvinova
Biomedicines.2023; 11(2): 396. CrossRef - Pharmacological Activation of Rev-erbα Attenuates Doxorubicin-Induced Cardiotoxicity by PGC-1α Signaling Pathway
Runmei Zou, Shuo Wang, Hong Cai, Yuwen Wang, Cheng Wang, Vivek Pandey
Cardiovascular Therapeutics.2023; 2023: 1. CrossRef - Protective Effect of Ergothioneine against 7-Ketocholesterol-Induced Mitochondrial Damage in hCMEC/D3 Human Brain Endothelial Cells
Damien Meng-Kiat Leow, Irwin Kee-Mun Cheah, Zachary Wei-Jie Fong, Barry Halliwell, Wei-Yi Ong
International Journal of Molecular Sciences.2023; 24(6): 5498. CrossRef - Effect of PPARγ on oxidative stress in diabetes-related dry eye
Jing Wang, Shuangping Chen, Xiuxiu Zhao, Qian Guo, Ruibo Yang, Chen Zhang, Yue Huang, Lechong Ma, Shaozhen Zhao
Experimental Eye Research.2023; 231: 109498. CrossRef - Chiisanoside Mediates the Parkin/ZNF746/PGC-1α Axis by Downregulating MiR-181a to Improve Mitochondrial Biogenesis in 6-OHDA-Caused Neurotoxicity Models In Vitro and In Vivo: Suggestions for Prevention of Parkinson’s Disease
Yu-Ling Hsu, Hui-Jye Chen, Jia-Xin Gao, Ming-Yang Yang, Ru-Huei Fu
Antioxidants.2023; 12(9): 1782. CrossRef - TBBPA causes apoptosis in grass carp hepatocytes involving destroyed ER-mitochondrial function
Dongxu Han, Naixi Yang, Huanyi Liu, Yujie Yao, Shiwen Xu
Chemosphere.2023; 341: 139974. CrossRef - The Protective Mechanism of TFAM on Mitochondrial DNA and its Role in Neurodegenerative Diseases
Ying Song, Wenjun Wang, Beibei Wang, Qiwen Shi
Molecular Neurobiology.2023;[Epub] CrossRef - Impact of Roux-en-Y Gastric Bypass on Mitochondrial Biogenesis and Dynamics in Leukocytes of Obese Women
Zaida Abad-Jiménez, Teresa Vezza, Sandra López-Domènech, Meylin Fernández-Reyes, Francisco Canet, Carlos Morillas, Segundo Ángel Gómez-Abril, Celia Bañuls, Víctor M. Víctor, Milagros Rocha
Antioxidants.2022; 11(7): 1302. CrossRef - The Effects of Galgunhwanggumhwangryun-tang on Glucose and Energy Metabolism in C2C12 Myotubes
Jihong Oh, Song-Yi Han, Soo Kyoung Lim, Hojun Kim
Journal of Korean Medicine for Obesity Research.2022; 22(2): 93. CrossRef
- Targeted metabolomics reveals the aberrant energy status in diabetic peripheral neuropathy and the neuroprotective mechanism of traditional Chinese medicine JinMaiTong
- Angiotensin II Inhibits Insulin Binding to Endothelial Cells
- Su-Jin Oh, Won-Chul Ha, Jee-In Lee, Tae-Seo Sohn, Ji-Hyun Kim, Jung-Min Lee, Sang-Ah Chang, Oak-Kee Hong, Hyun-Shik Son
- Diabetes Metab J. 2011;35(3):243-247. Published online June 30, 2011
- DOI: https://doi.org/10.4093/dmj.2011.35.3.243
- 3,828 View
- 24 Download
- 6 Crossref
-
 Abstract
Abstract
 PDF
PDF PubReader
PubReader Background Insulin-mediated glucose uptake in insulin target tissues is correlated with interstitial insulin concentration, rather than plasma insulin concentration. Therefore, insulin delivery to the interstitium of target tissues is very important, and the endothelium may also play an important role in the development of insulin resistance.
Methods After treating bovine aortic endothelial cells with angiotensin II (ATII), we observed the changes in insulin binding capacity and the amounts of insulin receptor (IR) on the cell membranes and in the cytosol.
Results After treatment of 10-7M ATII, insulin binding was decreased progressively, up to 60% at 60 minutes (
P <0.05). ATII receptor blocker (eprosartan) dose dependently improved the insulin binding capacity which was reduced by ATII (P <0.05). At 200 µM, eprosartan fully restored insulin binding capacity, althogh it resulted in only a 20% to 30% restoration at the therapeutic concentration. ATII did not affect the total amount of IR, but it did reduce the amount of IR on the plasma membrane and increased that in the cytosol.Conclusion ATII decreased the insulin binding capacity of the tested cells. ATII did not affect the total amount of IR but did decrease the amount of IR on the plasma membrane. Our data indicate that ATII decreases insulin binding by translocating IR from the plasma membrane to the cytosol. The binding of insulin to IR is important for insulin-induced vasodilation and transendothelial insulin transport. Therefore, ATII may cause insulin resistance through this endothelium-based mechanism.
-
Citations
Citations to this article as recorded by- Acute, local infusion of angiotensin II impairs microvascular and metabolic insulin sensitivity in skeletal muscle
Dino Premilovac, Emily Attrill, Stephen Rattigan, Stephen M Richards, Jeonga Kim, Michelle A Keske
Cardiovascular Research.2019; 115(3): 590. CrossRef - Angiotensin II type 2 receptor inhibits expression and function of insulin receptor in rat renal proximal tubule cells
Yang Yang, Caiyu Chen, Chunjiang Fu, Zaicheng Xu, Cong Lan, Yongchun Zeng, Zhi Chen, Pedro A. Jose, Ye Zhang, Chunyu Zeng
Journal of the American Society of Hypertension.2018; 12(2): 135. CrossRef - Endothelial function, its relation to arterial hypertension and the possibility of its modulation
Vladislav Biel, Jan Novák, Luděk Pluháček, Jiří Špác
Vnitřní lékařství.2018; 64(7-8): 762. CrossRef - Evidence to Consider Angiotensin II Receptor Blockers for the Treatment of Early Alzheimer’s Disease
Juan M. Saavedra
Cellular and Molecular Neurobiology.2016; 36(2): 259. CrossRef - Ameliorative effect of eprosartan on high-fat diet/streptozotocin-induced early diabetic nephropathy in rats
Mohamed A. Morsy, Gehan H. Heeba, Magda E. Mahmoud
European Journal of Pharmacology.2015; 750: 90. CrossRef - Metabolic actions of angiotensin II and insulin: A microvascular endothelial balancing act
Ranganath Muniyappa, Sahzene Yavuz
Molecular and Cellular Endocrinology.2013; 378(1-2): 59. CrossRef
- Acute, local infusion of angiotensin II impairs microvascular and metabolic insulin sensitivity in skeletal muscle

 KDA
KDA
 First
First Prev
Prev





